Last week Bakul Patel, an FDA senior policy advisor, highlighted the agency’s recent move to issue voluntary interoperability standards for medical devices. The changes include new standards for point-of-care and self-monitoring device technologies. Devices that adopt the standards will likely have an easier time attaining FDA approvals. This is on its surface an exciting and very positive development.
The promised benefits of device interoperability have been foreseen for quite some time, and yet device manufacturers to date have been frustratingly reluctant to make their products interoperable with other manufacturer’s products or hospitals’ health IT technology. Why the reluctance if the value proposition to the health care system is so compelling? The theories of disruptive innovation help explain why the past has played out in such a fragmented fashion and what the future likely holds for device interoperability.
Interdependence verses Modularity
The secret lies in the interplay between interdependent and modular product architectures (see graphic below). 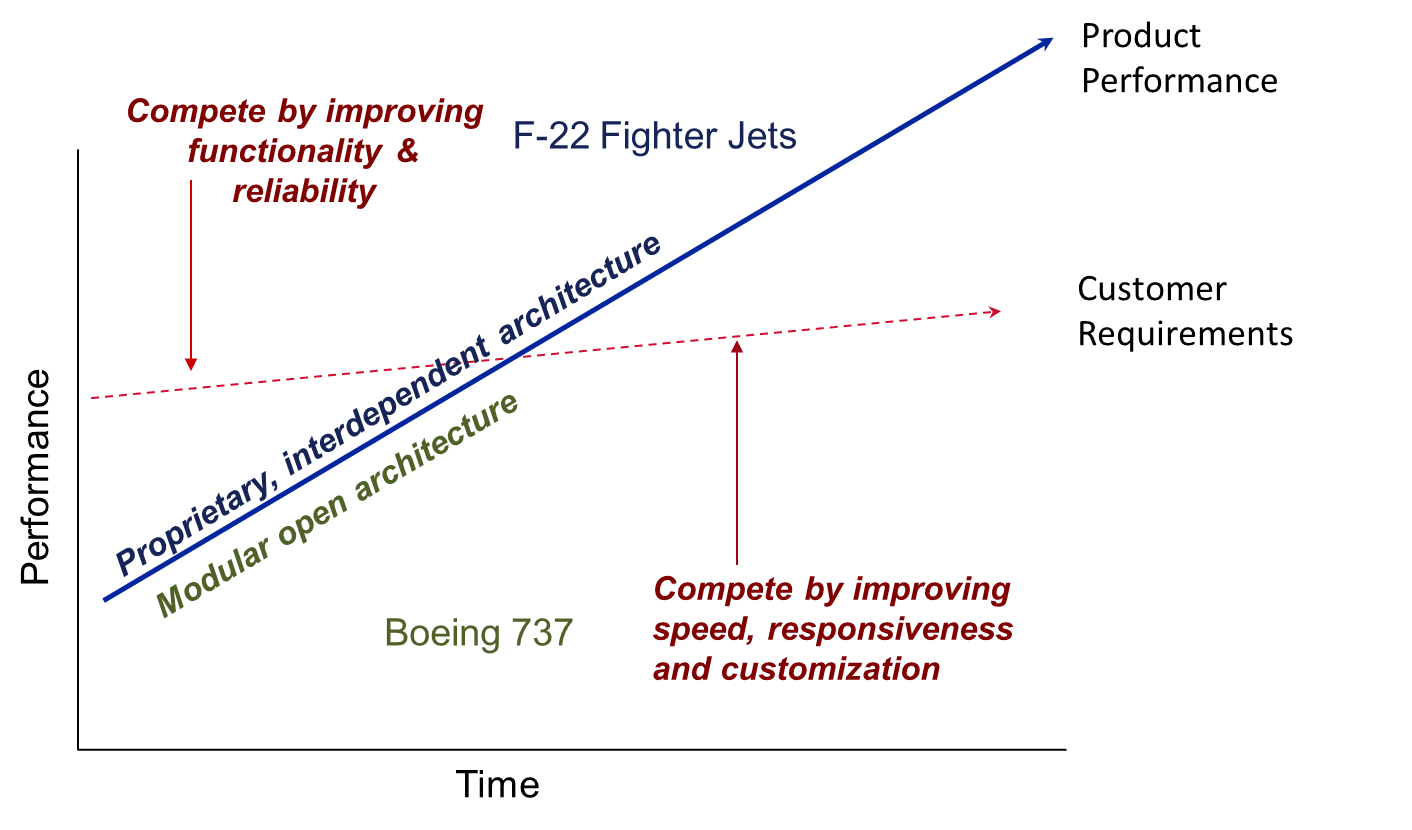 When a product is first developed, there are unpredictable interdependencies amongst the parts. The materials need to fit the design, the design needs to use the appropriate materials, and each piece must work together as a well-oiled machine (literally). For performance-focused products like this, the company usually needs to completely control the design and production of every interdependent component or else risk manufacturing surprises and performance issues. When performance isn’t as good as customers require, companies with interdependent product architectures make the most money.
When a product is first developed, there are unpredictable interdependencies amongst the parts. The materials need to fit the design, the design needs to use the appropriate materials, and each piece must work together as a well-oiled machine (literally). For performance-focused products like this, the company usually needs to completely control the design and production of every interdependent component or else risk manufacturing surprises and performance issues. When performance isn’t as good as customers require, companies with interdependent product architectures make the most money.
New high-tech military aircraft like Lockheed Martin’s F-22 fighter jet are examples of this type of product. This custom designed and assembled aircraft required the best engineers in the world working together in concert to manage the unknowns that inevitably arose during the process of creating a new high-performance machine. The final product is the best-performing fighter jet in the world, necessarily highly customized and not interoperable with any other aircraft design or maintenance scheme.
Most products begin like the F-22 fighter jet. Over time, however, product performance improves and eventually exceeds the customer’s requirements. When this happens, the basis of competition flips from performance and reliability to speed and flexibility. Design, manufacturing, and component performance become more routine and predictable. Once the basis of competition becomes speed and flexibility, products need to have modular architectures where the interfaces between components are standardized and flexibly used with multiple other components. In situations like this, an interdependent product architecture’s strengths become its weaknesses almost overnight! The money in these markets flows to the companies that define the standards and control the scarce, highest-performing component.
The Boeing 737 aircraft is an example of a product with modular architecture. It is built using standard components that have clearly specified interfaces and can be custom manufactured very quickly (in aircraft terms!). Software developers and avionics manufacturers have captured much of the profit in this industry in recent years because they are the most scarce, high-performing technology in the stack of standardized components – a completely different locus of value than in the F-22 fighter jet.
Implications for Medical Devices
What does this theory portend for medical device interoperability?
Demanding interoperability will require trade-offs in performance. If doctors, hospitals, and patients want interoperability, they’re saying they value speed and flexibility over continued increase in technical performance. This may be completely appropriate for technologies that are already good enough for most patients and doctors (e.g., thermometers, pulse oximeters, blood pressure monitors), but may not be true for the most novel technologies (e.g., new imaging modalities). Blanket statements that all technologies need to be high performing and interoperable immediately are a bit naïve. It is nearly impossible to design a modular product that represents a dramatic leap forward in technology performance.
Boeing’s 787 Dreamliner is an example of the tortuous schedule and budget overruns that await anyone that tries to develop a new technology to compete on performance and reliability with a modular product architecture. Doctors and hospitals will have to agree that the technologies which they want to be interoperable are good enough, and that they value speed and flexibility over continued increased technological performance. Otherwise, they’ll end up with systems integration projects not unlike the Dreamliner on their hands—projects that will be tremendously expensive and will likely deliver disappointing results.
Health systems need to start demanding solutions, not products. We wouldn’t expect an airline to purchase all of the component parts for an aircraft and assemble it themselves. Yet hospitals have historically been content to purchase and assemble all of the component parts for their health technology infrastructure themselves. We encourage health systems to begin to clearly articulate the technologies that are good enough and demand system solutions, not point solutions, from their device and technology vendors. The FDA has given one example of standards. Hospitals and health systems might be wise to articulate to their vendors that those standards are good enough for them and begin demanding solutions, not just products.
The exciting vision of device interoperability will happen in health care. Just don’t expect health care to be exempt from the way it has worked in every other industry to date.


